Antibody drug conjugates (ADCs) are molecules in which a chemotherapy drug is bound to an antibody and is delivered to the cancer cell very specifically; without damaging normal body cells. Nowadays various ADCs are available for therapeutic use in breast cancer, lung cancer, urinary bladder cancer etc.
Here we present a case of metastatic carcinoma breast treated with a ADC - Trastuzumab deruxtecan.
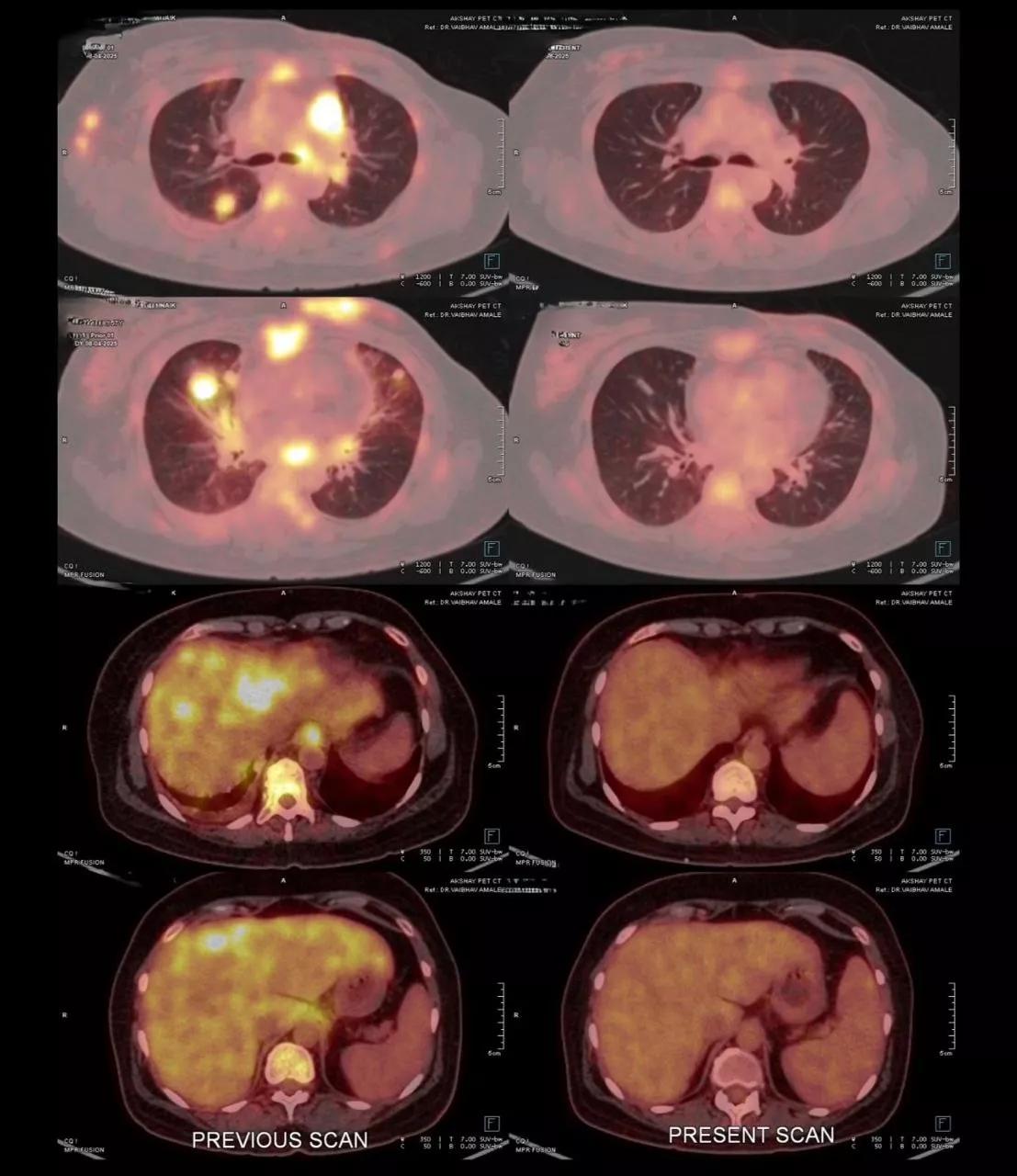
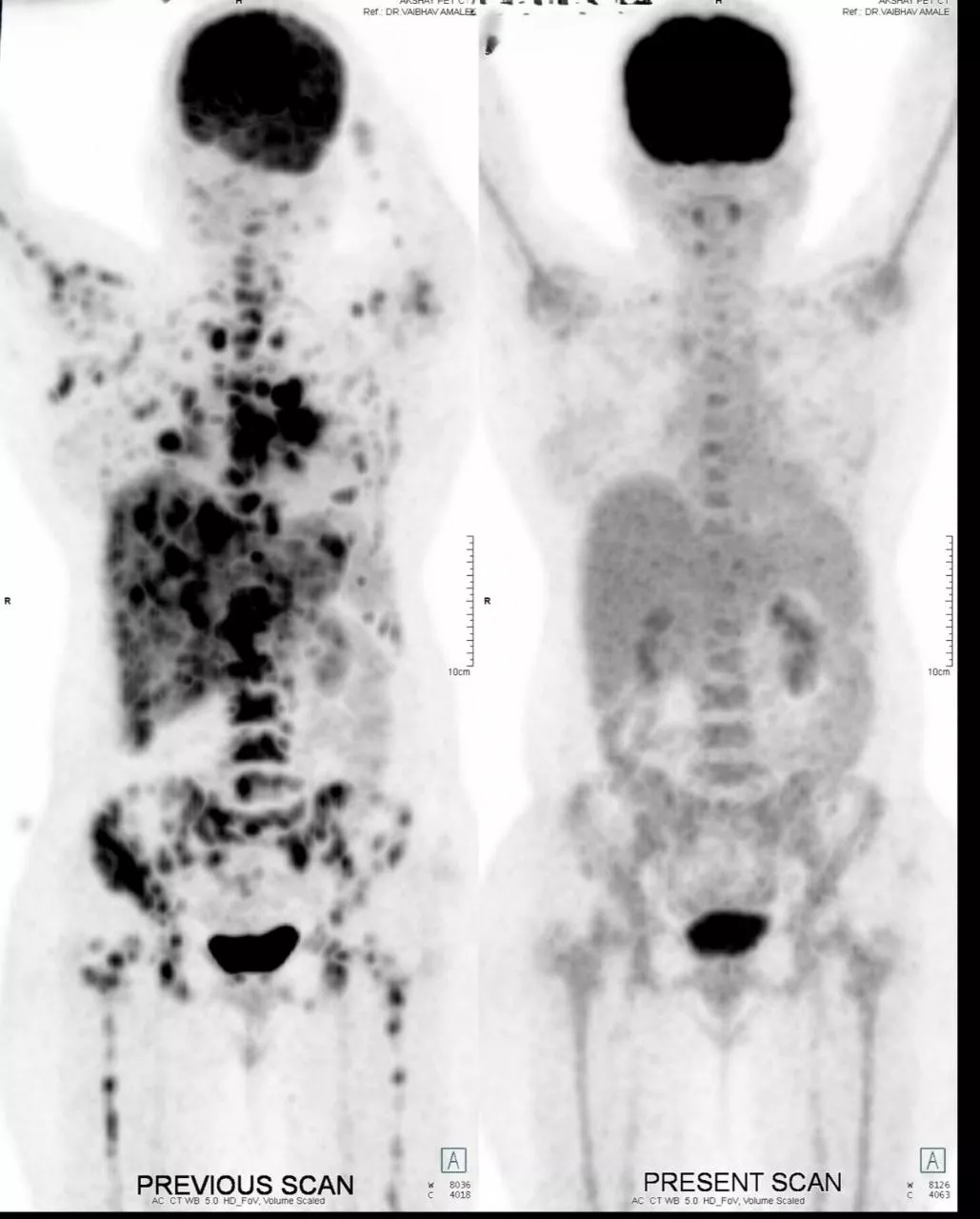
Patient is a 50 year female, Her2 positive metastatic breast cancer. She was diagnosed 2 years back with breast cancer. On diagnosis she was having lung metastatis. She received first line therapy with Paclitaxel and Trastuzumab. After one and half years, she had progression. She received second line treatment with Cap. Lapatinib and Tab. Capecitabine. She responded for 6 months. After 6 months she again progressed with new liver lesions, chest wall lesions and brain lesions. She was symptomatic for brain metastasis- headache, fatigue. She had mild derangement of liver function with elevated SGPT and SGOT, tender hepatomegaly. At this time we treated her with Inj. Trastuzumab Deruxtecan every 21 days. After 1 cycle she showed clinical improvement with reduced headaches and abdominal pain. After 2nd cycle she was almost asymptomatic, LFTs normalised. After 6 cycles, PET scan is showing Complete metabolic response. Brain lesions are metabolically inactive - resolved. She is currently asymptomatic and continuing same treatment.
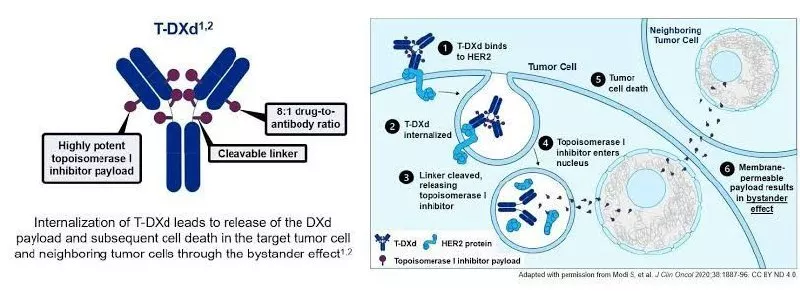
Trastuzumab Deruxtecan: It is a combination of trastuzumab and chemotherapeutic agent - Deruxtecan. Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate (ADC) that targets HER2-expressing cancer cells by binding to HER2 receptors on the cell surface, leading to internalization. Inside the cell, a cleavable linker releases the potent topoisomerase I inhibitor, deruxtecan (DXd), which then damages cancer cell DNA, inducing apoptosis. A unique feature is the bystander effect, where DXd's high membrane permeability allows it to kill nearby cancer cells, even those with low ( Her2 - 1 & 2) or no HER2 expression. This is in contrast to conventional Trastuzumab; where it works only in Her 2 positive ( 3 - High expression) tumors only.
Indications: 1. Breast cancer - Her 2 positive 2. Lung cancer- Her 2 positive 3. Stomach cancer - Her 2 positive
Dose: 5.4 mg per kg body weight as infusion.
Adverse effects: nausea, fatigue, vomiting, alopecia, constipation, decreased appetite, anemia, neutropenia, diarrhea, leukopenia, cough, and thrombocytopenia.
Specific side effect is Lung toxicity- Interstitial lung disease/ pneumonitis. It is seen in around 10 percent of treated patients, with a median time to onset of 5-6 months and an overall rate of fatal events of 2.2 percent. Asking the patient to report any respiratory symptoms at the earliest is advised. Even for asymptomatic patients, HRCT chest every 2 months is done. If any signs suggestive of ILD, treatment discontinuation is recommended.
Availability of newer ADCs is changing treatment paradigm of cancer. ADCs are now being used in earlier cancer stages also. Various molecules are available and are being used routinely in clinical practice.