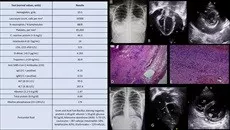
A 13-year-old girl came with a history of intermittent high-grade fever for 1 week, nausea and vomiting for 3 days, and swelling of hands and feet for 2 days. She had a history of SARS-CoV-2 exposure 1 month back but was asymptomatic then. On admission, she was febrile, tachypneic, tachycardiac, with a regular pulse that varied in intensity with the respiratory cycle, concerning for pulsus paradoxus. She had hypotension, engorged neck veins, facial puffiness, and edema over the hands and feet. On auscultation, air entry was decreased bilaterally in both lower zones, and heart sounds were distant. There was no murmur. The liver was palpable, and the umbilicus was everted. Her reverse transcriptase polymerase chain reaction test for SARS-CoV-2 was negative on admission, and anti–SARS-CoV-2 IgG antibodies were positive. Blood investigations showed increased inflammatory markers (Fig.). Chest radiogram showed cardiomegaly and blunted costophrenic angles bilaterally.
Right-hand panel showing investigations. A, Chest radiogram on admission; apical 4-chamber view (B) and parasternal short-axis view (C) on transthoracic echocardiogram at admission showing massive pericardial effusion (PE) with diastolic collapse of the right atrium (RA) and right ventricle (RV). Scanner view (D) and 40× view (E) of pericardial biopsy showing capillary congestion and leucocyte infiltration (inflammatory reaction). F, Chest radiogram at discharge. Apical 4-chamber view (G) and parasternal short-axis view (H) on transthoracic echocardiogram at discharge showing no pericardial collection. ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; COI, cut-off index; LA, left atrium; LDH, lactate dehydrogenase; LV, left ventricle; P, pericardium; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Echocardiography showed a structurally normal heart but massive pericardial effusion with right atrial and ventricular free wall collapse in diastole, normal biventricular function, and bilateral pleural effusion. In view of both pleural and pericardial effusions, she underwent urgent surgical “wide anterior pericardiectomy” with bilateral intercostal tube drainage, and 650 mL of transudative pericardial fluid was drained. The epicardium and pericardium were intense red colored, and pericardial biopsy confirmed acute inflammation. She received intravenous immunoglobulin and high-dose intravenous steroids and showed clinical and hemodynamic improvement within 48 hours. She was discharged after 1 week of drain removal, on tapering doses of oral steroids and aspirin. At 1-month follow-up, she was asymptomatic, and there was no pericardial collection on echocardiography evaluation.
Pericardial involvement in acute coronavirus disease 2019 may not always be associated with myocardial involvement and can rarely present as tamponade requiring drainage. Pericardial involvement in MIS-C has also been known; however, in a recent study, only 20% of children presenting with acute cardiovascular manifestations had associated mild-to-moderate pericardial effusion on echocardiogram, and none had severe effusion. MIS-C presenting with pericardial tamponade requiring emergency pericardiocentesis has not been reported. Our case not only shows a rare presentation of MIS-C but also highlights the possibility of surgical intervention in the management of MIS-C. Shock in MIS-C can have multiple reasons, of which, although rare, pericardial tamponade should also be considered.